Amniotic Membrane Market
Amniotic Membrane Market by application (ophthalmology, dermatology, otolaryngology, surgical wound, orthopedics, and others), by type (dehydrated and cryopreserved), by end-user (ambulatory surgical center, hospital and clinic and others) - Global Industry Share, Growth, Competitive Analysis and Forecast, 2019-2025 Update Available - Forecast 2025-2035
Amniotic Membrane Market is expected to grow at a considerable rate during the forecast period 2019-2025. Amniotic membrane (AM) is a fetal membrane that is harvested from placental tissue. The placental tissue contains healing properties that support in ocular surface repair. Amniotic membrane is intended for use in particular conjunctival and corneal diseases. Its uses comprise prevention of ocular complications concerned with conjunctival forniceal reconstruction, primary/recurrent pterygia and Stevens-Johnson syndrome. Additionally, AM supports to prevent scar tissue formation and wound healing as it holds potential anti-scarring and antifibrotic properties. The factors that are contributing towards the growth of the market include rising adoption of stem cell therapy and FDA approvals for AM products.
Additionally, the increasing prevalence of vision impairment and blindness is the major factor encouraging the demand for AM in ophthalmic surgeries. According to the World Health Organization (WHO), it is estimated that nearly 1.3 billion people across the globe are suffering from some kind of vision impairment. Nearly 188.5 million people are suffering from mild vision impairment and 217 million people with moderate and severe vision impairment and 36 million people are blind. The key causes of vision impairment across the globe comprise cataract, uncorrected refractive errors, age-related macular degeneration, diabetic neuropathy, glaucoma, corneal opacity and trachoma. This rising incidence of vision impairment has significantly increased the demand for ophthalmic surgeries. For instance, according to Eurostat, cataract surgery was performed 4.5 million times across the EU member states in 2016. Portugal had the highest rate of cataract operations in Europe. The number of cataract operations performed in the country was 14 surgeries per thousand inhabitants. It was followed by France and Austria, with 13 cataract operations per thousand inhabitants. This trend towards cataract surgeries is creating a significant opportunity for the market. Over the last few years, AM has become a tool in ocular surface optimization earlier to the ophthalmic procedure, primarily as a premium intraocular lens (IOLs). It can be used by the surgeon to perform refractive cataract surgery for patients with dry eye. AM devices enable surgeons to firstly restore the health of ocular surface earlier performing refractive cataract surgery.
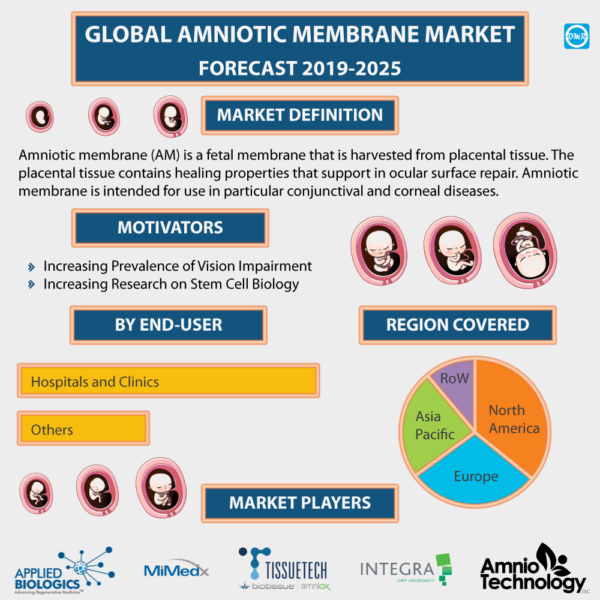
Moreover, the global amniotic membrane market is segmented on the basis of application, type and end-user. On the basis of application, the market is further classified into ophthalmology, dermatology, otolaryngology, surgical wound, orthopedics and others. On the basis of type, the market is further classified into dehydrated and cryopreserved. Cryopreservation of AM encompasses slow freezing at -80 degree Celsius with the use of DMEM/glycerol preservation media that enables slow rate freezing without formation of ice. One of the major instances of the cryopreserved form of AM includes ProKera by BioTissue. This is inserted into the eye like a contact lens placement and requires no assembly. Moreover, dehydrated AM is preserved with the use of vacuum with low-temperature heat. This allows retaining devitalized cellular components. As compared to cryopreservation tissue, dehydrated AM is preserved at room temperature; however, it needs to be rehydrated for clinical use. AmbioDisk by IOPOphthalmics is an instance of dehydrated AM for in-office use, which is applied directly to the ocular surface. It is covered with a bandage contact lens. On the basis of end-user, the market is further classified into ambulatory surgical centers, hospitals and clinics and others.
Geographically, the global amniotic membrane market is classified into North America, Europe, Asia-Pacific and Rest of the World (RoW). North America has been witnessing potential growth in the market due to the significant adoption of stem cell therapy and well-developed healthcare infrastructure in the region. However, Asia-pacific is estimated to witness robust growth during the forecast period, due to the increasing prevalence of ophthalmic diseases in the region.
The major players involved in the market comprise Integra LifeSciences Corp., BioStem Technologies Inc., Applied Biologics LLC, MiMedx Group Inc., and Katena Products Inc. The key strategies adopted by the major players to increase market share globally include merger and acquisitions, product launch and partnerships, and collaborations. For instance, in October 2018, BioStem LifeSciences, a subsidiary of BioStem Technologies Inc., introduced four amniotic membrane products. These products are primarily an amniotic tissue allograft membrane with chorion layer, an amniotic tissue allograft flowable matrix, a chorion-free amniotic tissue allograft membrane and an extracellular amniotic membrane allograft for ocular repair. Such products are designed for multiple indications within orthopedics, advanced wound care and ophthalmology markets.
Research Methodology
The market study of global amniotic membrane market is incorporated by extensive primary and secondary research conducted by research team at OMR. Secondary research has been conducted to refine the available data to breakdown the market in various segments, derive total market size, market forecast and growth rate. Different approaches have been worked on to derive the market value and market growth rate. Our team collects facts and data related to the market from different geography to provide a better regional outlook. In the report country level analysis is provided by analyzing various regional players, regional tax laws and policies, consumer behavior and macro-economic factors. Numbers extracted from secondary research have been authenticated by conducting proper primary research. It includes tracking down key people from the industry and interviewing them to validate the data. This enables our analyst to derive the closest possible figures without any major deviations in the actual number. Our analysts try to contact as many executives, managers, key opinion leaders and industry experts. Primary research brings the authenticity in our reports.
Secondary Sources Include
- Financial reports of companies involved in the market
- Authentic Public Databases such as WHO, American Academy of Ophthalmology and so on.
- Whitepapers, research-papers, and news blogs
- Company websites and their product catalogue
The report is intended for government and private companies for overall market analysis and competitive analysis. The report provides in-depth analysis on pricing, market size, intended quality of the product preferred by consumers and initial norms. The report will serve as a source for 360-degree analysis of the market thoroughly integrating different models.
Market Segmentation
Global amniotic membrane market is segmented on the basis of regional outlook and following segments:
- Global Amniotic Membrane Market Research and Analysis, By Application
- Global Amniotic Membrane Market Research and Analysis, By Type
- Global Amniotic Membrane Market Research and Analysis, By End-User
- Global Amniotic Membrane Market Research and Analysis, By Region
The Report Covers
- Comprehensive research methodology of the global amniotic membrane market.
- This report also includes detailed and extensive market overview with key analyst insights.
- Exhaustive analysis of macro and micro factors influencing the market guided by key recommendations.
- Analysis of regional regulations and other government policies impacting the global amniotic membrane market.
- Insights about market determinants which are stimulating the global amniotic membrane market.
- Detailed and extensive market segments with regional distribution of forecasted revenues.
- Extensive profiles and recent developments of market players.
1. Report Summary
1.1. Research Methods and Tools
1.2. Market Breakdown
1.2.1. By Segments
1.2.2. By Geography
1.2.3. By Stakeholders
2. Market Overview and Insights
2.1. Scope of the Report
2.2. Analyst Insight & Current Market Trends
2.2.1. Key Findings
2.2.2. Recommendations
2.2.3. Conclusion
2.3. Regulations
3. Competitive Landscape
3.1. Competitive Dashboard
3.2. Key Strategy Analysis
3.3. Impact Analysis
3.4. Key Company Analysis
3.4.1. Integra LifeSciences Corp.
3.4.1.1. Overview
3.4.1.2. Financial Analysis
3.4.1.3. SWOT Analysis
3.4.1.4. Recent Developments
3.4.2. TissueTech, Inc.
3.4.2.1. Overview
3.4.2.2. Financial Analysis
3.4.2.3. SWOT Analysis
3.4.2.4. Recent Developments
3.4.3. MiMedx Group, Inc.
3.4.3.1. Overview
3.4.3.2. Financial Analysis
3.4.3.3. SWOT Analysis
3.4.3.4. Recent Developments
3.4.4. Applied Biologics, LLC
3.4.4.1. Overview
3.4.4.2. Financial Analysis
3.4.4.3. SWOT Analysis
3.4.4.4. Recent Developments
3.4.5. Amnio Technology, LLC
3.4.5.1. Overview
3.4.5.2. Financial Analysis
3.4.5.3. SWOT Analysis
3.4.5.4. Recent Developments
4. Market Determinants
4.1. Motivators
4.2. Restraints
4.3. Opportunities
5. Market Segmentation
5.1. Global Amniotic Membrane Market by Application
5.1.1. Ophthalmology
5.1.2. Dermatology
5.1.3. Otolaryngology
5.1.4. Surgical Wound
5.1.5. Orthopedic
5.1.6. Others (Genitourinary Reconstruction)
5.2. Global Amniotic Membrane Market by Type
5.2.1. Dehydrated
5.2.2. Cryopreserved
5.3. Global Amniotic Membrane Market by End-User
5.3.1. Ambulatory Surgical Centers
5.3.2. Hospitals and Clinics
5.3.3. Others (Research Institutes)
6. Regional Analysis
6.1. North America
6.1.1. United States
6.1.2. Canada
6.2. Europe
6.2.1. UK
6.2.2. Germany
6.2.3. Italy
6.2.4. Spain
6.2.5. France
6.2.6. Rest of Europe
6.3. Asia-Pacific
6.3.1. China
6.3.2. India
6.3.3. Japan
6.3.4. Rest of Asia-Pacific
6.4. Rest of the World
7. Company Profiles
7.1. Alliqua BioMedical, Inc.
7.2. Amnio Technology, LLC
7.3. Applied Biologics, LLC
7.4. AxoGen, Inc.
7.5. Axolotl Biologix, Inc.
7.6. BioStem Technologies, Inc.
7.7. FzioMed, Inc.
7.8. Human Regenerative Technologies, LLC
7.9. Integra LifeSciences Corp.
7.10. Katena Products, Inc.
7.11. MiMedx Group, Inc.
7.12. Orthofix Holdings, Inc.
7.13. Osiris Therapeutics, Inc.
7.14. Predictive Biotech, Inc.
7.15. Seed Biotech, Inc.
7.16. Skye Biologics, Inc.
7.17. Surgenex, LLC
7.18. SurgiLogix, LLC.
7.19. TissueTech, Inc.
7.20. Vivex Biomedical, Inc.
7.21. XCell Biologix


