Tuberculosis Diagnosis and Treatment Market Size
Global Tuberculosis Diagnosis and Treatment Market Size, Share & Trends Analysis Report, By Diagnosis (Laboratory Testing, Nucleic Acid Tests, Radiography, Drug Susceptibility Test, and Other Tests),B Drugs (First Line Drugs and Second Line Drugs), By End-User (Hospitals& Clinics, Government & Non-Government Organizations, and Others), and Forecast 2019-2025 Update Available - Forecast 2025-2035
The global tuberculosis diagnosis and treatment market are expected to exhibit a CAGR of 2.0% during the forecast period. The market growth is attributed to the increasing demand for technologically advanced testing procedures and the emergence of MDR-TB and XDR-TB strains for the treatment of tuberculosis. World Health Organization (WHO) also recommended a robust and rapid DNA-based test in 2016, which serves as an alternative to sputum smear microscopy. However, certain factors such as lack of awareness about the diagnosis and treatment methods in countries such as Africa have been acting as the major restraints for the market.
Moreover, increasing R&D activities to further advance TB diagnostics will create a future opportunity for the growth of the global tuberculosis diagnosis and treatment market. Considering the rising drug-resistant infections, the demand for new vaccines, faster-acting drugs, new diagnostic technologies, and R&D activities are on the rise.
Segmental Outlook
The global tuberculosis diagnosis and treatment market aresegmented on the basis of diagnosis, drugs, and end-user. Based on the diagnosis, the market is segmented into laboratory testing, nucleic acid tests, radiography, drug susceptibility test, and other tests.Laboratory testing for tuberculosis diagnosis is expected to hold a significant share in the market owing to increasing penetration of this test in the developing regions where there is high prevalence rate of tuberculosis. Based on the end-user, the market is segmented into hospitals& clinics, government & non-government organizations, and others.
Further, on the basis of drugs, the market is segmented into first-line drugs and second-line drugs. First-line drugs involve Pyrazinamide (Z/PZA), Ethambutol (E/EMB), Rifabutin (RFB), Rifapentine, and Streptomycin. In addition, second-line drugs include injectable agents such as Kanamycin (KM), Amikacin (AMK), and Capreomycin (CM); oral bacteriostatic second-line agent such asTerizidone (TRD) and Ethionamide (ETO); and others.
Global Tuberculosis Diagnosis and Treatment Market Research and Analysis by Diagnosis, 2018
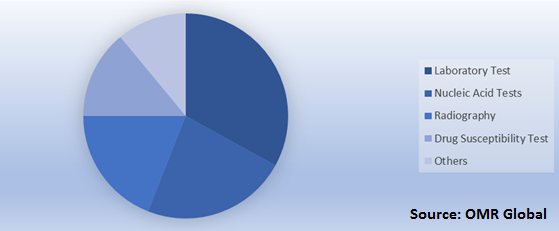
Laboratory Diagnosis Test Have a Significant Penetration in the Tuberculosis Diagnosis Industry
Laboratory tests include smear microscopy and culture-based tests. Additionally, these tests dominate the diagnostic segment due to their wide adoption in the industry. Besides, culture-based tests help diagnose and confirm active tuberculosis more accurately. Moreover, a culture test is one of the major confirmatory tests used for tuberculosis diagnosis that utilizes sputum or other clinical specimens and it is cost-efficient as compared to other tests. Furthermore, nucleic acid tests are expected to grow at a remarkable rate during the forecast period owing to continuous advancements in technology.
Regional Outlook
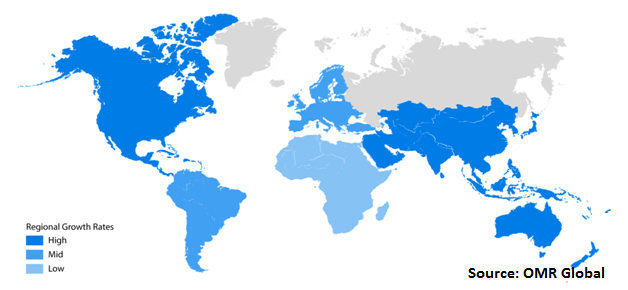
The global tuberculosis diagnosis and treatment market areanalyzed on the basis of the geographical regions that are contributing significantly towards the growth of the market. On the basis of geography, the market is segmentedinto North America, Europe, Asia-Pacific and Rest of the World. Asia-Pacificis expectedto contribute significantly to the growth of the global tuberculosis diagnosis and treatment industry.
Global Tuberculosis Diagnosis and Treatment Market Growth, by Region 2019-2025
Market Players Outlook
The global tuberculosis industry is studied on the basis of the tuberculosis diagnosis perspective as well as tuberculosis treatment perspective. Various players providing products for the diagnosis and treatment of tuberculosis have been studied in the report. Some of the key players operating in the tuberculosis diagnosis market include Abbott Laboratories, Inc., F. Hoffmann La Roche AG, and Beckton, Dickinson and Co. While, some of the major players providing tuberculosis drugs include Otsuka Pharmaceuticals Co. Ltd., Johnson & Johnson Services, Inc., and Novartis AG.
Apart from these, government and non-government organizations are contributing significantly to the market growth by bringing new drugs into the market. For instance, in august 2019, Pretomanid, developed by the non-profit TB Alliance, has received the FDA approval for the treatment of some of the most drug-resistant forms of tuberculosis (TB). This drug was approved under the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD pathway) for the treatment of people with extensively drug-resistant TB (XDR-TB) or multidrug-resistant TB (MDR-TB) who are treatment-intolerant.
The Report Covers
- Market value data analysis of 2018 and forecast to 2025.
- Annualized market revenues ($ million) for each market segment.
- Country-wise analysis of major geographical regions.
- Key companies operating in the global tuberculosis diagnosis and treatment market. Based on the availability of data, information related to products, and relevant news is also available in the report.
- Analysis of business strategies by identifying the key market segments positioned for strong growth in the future.
- Analysis of market-entry and market expansion strategies.
- Competitive strategies by identifying ‘who-stands-where’ in the market.
1. Report Summary
1.1. Research Methods and Tools
1.2. Market Breakdown
1.2.1. By Segments
1.2.2. By Geography
2. Market Overview and Insights
2.1. Scope of the Report
2.2. Analyst Insight & Current Market Trends
2.2.1. Key Findings
2.2.2. Recommendations
2.2.3. Conclusion
2.3. Rules & Regulations
3. Competitive Landscape
3.1. Company Share Analysis
3.2. Key Strategy Analysis
3.3. Key Company Analysis
3.3.1. Otsuka Pharmaceuticals Co. Ltd.
3.3.1.1. Overview
3.3.1.2. Financial Analysis
3.3.1.3. SWOT Analysis
3.3.1.4. Recent Developments
3.3.2. Johnson & Johnson Services Inc.
3.3.2.1. Overview
3.3.2.2. Financial Analysis
3.3.2.3. SWOT Analysis
3.3.2.4. Recent Developments
3.3.3. Abbott Laboratories, Inc.
3.3.3.1. Overview
3.3.3.2. Financial Analysis
3.3.3.3. SWOT Analysis
3.3.3.4. Recent Developments
3.3.4. Becton, Dickinson and Co.
3.3.4.1. Overview
3.3.4.2. Financial Analysis
3.3.4.3. SWOT Analysis
3.3.4.4. Recent Developments
3.3.5. Thermo Fisher Scientific, Inc.
3.3.5.1. Overview
3.3.5.2. Financial Analysis
3.3.5.3. SWOT Analysis
3.3.5.4. Recent Developments
4. Market Determinants
4.1. Motivators
4.2. Restraints
4.3. Opportunities
5. Market Segmentation
5.1. Global Tuberculosis Diagnosis andTreatment Market by Diagnosis
5.1.1. Laboratory Testing
5.1.2. Nucleic Acid Tests
5.1.3. Radiography
5.1.4. Drug Susceptibility Test
5.1.5. Other Tests
5.2. Global Tuberculosis Diagnosis and Treatment Market by Drugs
5.2.1. First Line Drugs
5.2.2. Second Line Drugs
5.3. Global Tuberculosis Diagnosis andTreatment Market by End-User
5.3.1. Hospitals& Clinics
5.3.2. Government & Non-Government Organizations
5.3.3. Others
6. Regional Analysis
6.1. North America
6.1.1. US
6.1.2. Canada
6.2. Europe
6.2.1. UK
6.2.2. Germany
6.2.3. Italy
6.2.4. Spain
6.2.5. France
6.2.6. Rest of Europe
6.3. Asia-Pacific
6.3.1. China
6.3.2. India
6.3.3. Japan
6.3.4. Rest of Asia-Pacific
6.4. Rest of the World
7. Company Profiles
7.1. Abbott Laboratories Inc.
7.2. Akonni Biosystems, Inc.
7.3. Alere, Inc.
7.4. Becton Dickinson and Co.
7.5. Biochemical and Synthetic Products Pvt Ltd
7.6. Biomérieux SA
7.7. Cepheid Inc.
7.8. Eiken Chemical Co. Ltd.
7.9. EpistemLtd.
7.10. F. Hoffmann-LA Roche AG
7.11. GlaxoSmithKline PLC
7.12. Hain Lifescience GmbH
7.13. HologicInc.
7.14. Johnson & Johnson Services, Inc.
7.15. LabatecPharma SA
7.16. Lupin Pharmaceuticals Inc.
7.17. Novartis AG
7.18. Otsuka Pharmaceutical Co. Ltd.
7.19. PerkinElmer Inc.
7.20. QiagenGmbH
7.21. Sanofi SA
7.22. Thermo Fisher Scientific, Inc.
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DIAGNOSIS, 2018-2025 ($ MILLION)
- GLOBAL LABORATORY TESTINGFOR TUBERCULOSIS DIAGNOSIS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL NUCLEIC ACID TESTSFOR TUBERCULOSIS DIAGNOSIS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL RADIOGRAPHYFOR TUBERCULOSIS DIAGNOSIS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL DRUG SUSCEPTIBILITY TESTFOR TUBERCULOSIS DIAGNOSIS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL OTHER TESTSFOR TUBERCULOSIS DIAGNOSIS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DRUGS, 2018-2025 ($ MILLION)
- GLOBAL FIRST LINE DRUGSFOR TUBERCULOSIS TREATMENT MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL SECOND LINE DRUGSFOR TUBERCULOSIS TREATMENT MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY END-USER, 2018-2025 ($ MILLION)
- GLOBAL HOSPITALS& CLINICS MARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL GOVERNMENT & NON-GOVERNMENT ORGANIZATIONSMARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL OTHER END-USERSMARKET RESEARCH AND ANALYSIS BY REGION, 2018-2025 ($ MILLION)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY GEOGRAPHY, 2018-2025 ($ MILLION)
- NORTH AMERICANTUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET RESEARCH AND ANALYSIS BY COUNTRY, 2018-2025 ($ MILLION)
- NORTH AMERICANTUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET RESEARCH AND ANALYSIS BY DIAGNOSIS, 2018-2025 ($ MILLION)
- NORTH AMERICAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DRUGS, 2018-2025 ($ MILLION)
- NORTH AMERICAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY END-USER, 2018-2025 ($ MILLION)
- EUROPEANTUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY COUNTRY, 2018-2025 ($ MILLION)
- EUROPEAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DIAGNOSIS, 2018-2025 ($ MILLION)
- EUROPEAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DRUGS, 2018-2025 ($ MILLION)
- EUROPEAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY END-USER, 2018-2025 ($ MILLION)
- ASIA-PACIFICTUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET RESEARCH AND ANALYSIS BY COUNTRY, 2018-2025 ($ MILLION)
- ASIA-PACIFIC TUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET RESEARCH AND ANALYSIS BY DIAGNOSIS, 2018-2025 ($ MILLION)
- ASIA-PACIFIC TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DRUGS, 2018-2025 ($ MILLION)
- ASIA-PACIFIC TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY END-USER, 2018-2025 ($ MILLION)
- REST OF THE WORLD TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DIAGNOSIS, 2018-2025 ($ MILLION)
- REST OF THE WORLD TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY DRUGS, 2018-2025 ($ MILLION)
- REST OF THE WORLD TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET RESEARCH AND ANALYSIS BY END-USER, 2018-2025 ($ MILLION)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET SHARE BY DIAGNOSIS, 2018 VS 2025 (%)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENTMARKET SHARE BY DRUGS, 2018 VS 2025 (%)
- GLOBAL TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SHARE BY END-USER, 2018 VS 2025 (%)
- US TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- CANADA TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- THE UK TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- FRANCE TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- GERMANY TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- ITALY TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET, 2018-2025 ($ MILLION)
- SPAINTUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- ROETUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- INDIA TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- CHINA TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- JAPAN TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- REST OF ASIA-PACIFIC TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)
- REST OF THE WORLD TUBERCULOSIS DIAGNOSIS AND TREATMENT MARKET SIZE, 2018-2025 ($ MILLION)


